Selective carbon dioxide adsorption of ε-Keggin-type zincomolybdate-based purely inorganic 3D frameworks†
Abstract
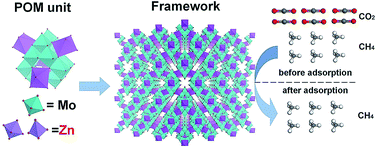
Polyoxometalate-based 3D frameworks, Na1.5H11.4[ZnMo12O40{Zn2}]·5.5H2O and (NH4)1.5H8.5[ZnMo12O40{Zn2}]·6H2O, are synthesized in moderate yields. Rotation of the reactor under hydrothermal conditions is essential to improve the yield. The materials show zeolite-like selective molecule adsorption properties. Depending on the micropore aperture size of the materials, small molecules can be adsorbed in the materials, while large molecules cannot. The enthalpy of adsorption and DFT calculation indicate that the materials strongly interact with CO2, but weakly interact with CH4, due to electrostatic interactions between the materials and molecules. CO2/CH4 co-sorption experiments show that the materials can selectively adsorb CO2, and CO2 adsorption selectivity of the material with sodium cations is higher than that of the material with ammonium cations. The material with sodium ions can be utilized for gas chromatographic separation of CH4 and CO2.

 Please wait while we load your content...
Please wait while we load your content...