Improved electrochemical performance of Na0.67MnO2 through Ni and Mg substitution†
Abstract
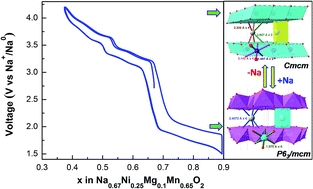
Incorporating nickel and magnesium into P2–Na0.67MnO2 is expected to suppress Jahn–Teller active Mn3+ ions, increase the average potential of the electrode through Ni4+/Ni2+ redox couples and stabilize M–O layers resulting in a smooth cycling profile with better capacity retention. In this communication, P2–Na0.67Ni0.25Mg0.1Mn0.65O2 is reported as the cathode material for rechargeable Na-ion batteries. It reversibly intercalates ∼0.52 moles of Na per formula unit in the voltage region of 1.5–4.2 V at C/10 rate which corresponds to a specific capacity of 140 mA h g−1. X-ray photoelectron spectroscopy has attributed the origin of the capacity in this phase to both Ni and Mn redox species active at different potential ranges. Substituting part of Mn with Ni and Mg increases the average potential of Na0.67MnO2 and improves the energy density of Na0.67Ni0.25Mg0.1Mn0.65O2 to 335 W h kg−1.

 Please wait while we load your content...
Please wait while we load your content...