Molecular engineering of benzothienoisoindigo copolymers allowing highly preferential face-on orientations†
Abstract
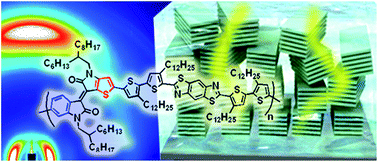
Orientation of conjugated polymers is increasingly important in organic photovoltaics (OPV) to achieve high power conversion efficiency (PCE). The optimized orientation of conjugated backbones for photo-generated charge carriers in OPV devices is in contrast to organic semiconductor devices, demanding new strategies to control and realize face-on orientation of conjugated systems onto substrates. Here we report new conjugated polymers composed of electron-accepting benzothienoisoindigo (BTIDG), an asymmetric unit of isoindigo and thienoisoindigo. BTIDG was coupled with weakly electron-donating thiazolothiazole or benzobisthiazole, concurrently leading to moderate optical band gaps (1.41–1.52 eV) and the highest occupied molecular orbital (−5.35 to −5.50 eV). The alkylthiophene spacer between BTIDG and the donor unit provided a marked control over the orientation of polymers, among which the degree of face-on orientation as high as 95% was revealed by grazing incidence X-ray diffraction. The maximum PCE was improved up to 4.2% using the system with [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM). We present a useful basis on the structure (orientation)–property (OPV output) relationship to lay down new guidelines for the design of efficient solar cell materials.


 Please wait while we load your content...
Please wait while we load your content...