Structural, electrochemical and magnetic properties of a novel KFeSO4F polymorph†
Abstract
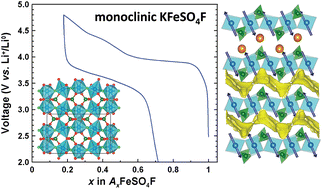
In the quest for sustainable and low-cost positive electrode materials for Li-ion batteries, we discovered, as reported herein, a new low temperature polymorph of KFeSO4F. Contrary to the high temperature phase crystallizing in a KTiOPO4-like structure, this new phase adopts a complex layer-like structure built on FeO4F2 octahedra and SO4 tetrahedra, with potassium cations located in between the layers, as solved using neutron and synchrotron diffraction experiments coupled with electron diffraction. The detailed analysis of the structure reveals an alternation of edge- and corner-shared FeO4F2 octahedra leading to a large monoclinic cell of 1771.774(7) Å3. The potassium atoms are mobile within the structure as deduced by ionic conductivity measurements and confirmed by the bond valence energy landscape approach thus enabling a partial electrochemical removal of K+ and uptake of Li+ at an average potential of 3.7 V vs. Li+/Li0. Finally, neutron diffraction experiments coupled with SQUID measurements reveal a long range antiferromagnetic ordering of the Fe2+ magnetic moments below 22 K with a possible magnetoelectric behavior.

 Please wait while we load your content...
Please wait while we load your content...