Synthesis and energetic properties of high-nitrogen substituted bishomocubanes†
Abstract
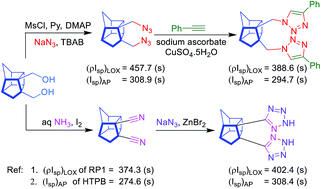
Synthesis, thermodynamic characterization, and energetic properties of three novel high-nitrogen bishomocubane-based compounds DADMBHC, DTetzBHC and DPTrizDMBHC are reported here. These compounds have higher heats of formation (HoFs) and higher energy densities as compared to traditional hydrocarbon fuels. Densities, gas phase HoF and their optimized molecular structure geometries were calculated with various levels of theory. In general, the calculated HoFs of these compounds turn out to be extremely high. Ballistic properties such as vacuum specific impulse and density vacuum specific impulse were calculated using the NASA Chemical Equilibrium and Applications utility. Propulsive properties were compared with liquid bipropellants (RP1) and solid propellants (AP) and explosive properties were compared with RDX. The density specific impulse demonstrated an improvement of 35 s for DADMBHC and DTetzBHC over standard liquid hydrocarbon HTPB, thus showing promise as possible monomers to replace HTPB as a fuel-binder. The density specific impulses of these compounds were also found to be significantly higher than that of RP1, e.g. that of DADMBHC was found to be higher by 84 s, making them potentially good candidates as propellants for use under volume-limited conditions. The detonation properties showed that these compounds have low potential as explosives. TGA, coupled with IR spectroscopy, revealed that DADMBHC and DPTrizDMBHC evaporate readily while DTetzBHC decomposes partially.


 Please wait while we load your content...
Please wait while we load your content...