Ultra low band gap α,β-unsubstituted BODIPY-based copolymer synthesized by palladium catalyzed cross-coupling polymerization for near infrared organic photovoltaics†
Abstract
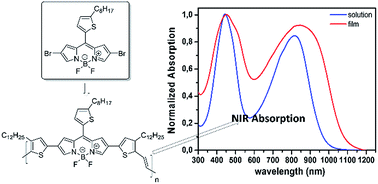
A new ultra low band gap (LBG) α,β-unsubstituted BODIPY-based conjugated polymer has been synthesized by conventional cross coupling polymerization techniques (Stille cross coupling) for the first time. The polymer exhibits a panchromatic absorption spectrum ranging from 300 nm to 1100 nm and an optical band gap (Eoptg) of 1.15 eV, suitable for near infrared (NIR) organic photovoltaic applications as electron donor. Preliminary power conversion efficiency (PCE) of 1.1% in polymer : [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) 1 : 3 weight ratio bulk heterojunction (BHJ) solar cells has been achieved, demonstrating very interesting and promising photovoltaic characteristics, such as good fill factor (FF) and open circuit voltage (Voc). These results showing that by the proper chemical design, new α,β-unsubstituted BODIPY-based NIR copolymers can be developed in the future with suitable energy levels matching those of PC71BM towards more efficient NIR organic photovoltaics (OPVs).

 Please wait while we load your content...
Please wait while we load your content...