Effect of solvents on the growth of TiO2 nanorods and their perovskite solar cells
Abstract
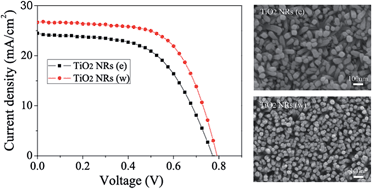
TiO2 nanorods were synthesized by hydrothermal methods with ethanol–HCl and water–HCl solutions, respectively, and CH3NH3PbI3−xClx perovskite solar cells based on them were fabricated. The power conversion efficiency (PCE) of the best solar cells based on TiO2 nanorods with water–HCl solution is higher than that with ethanol–HCl solution. The dimensions, morphology, optical properties, and photogenerated charge behavior of the two kinds of samples were investigated. The results indicate that the better performance of solar cells based on TiO2 nanorods with water–HCl solution than those with ethanol–HCl solution could be attributed to their special orientation, high conductivity, improved morphology, good optical properties, fast charge transfer and reduced charge recombination. A PCE of 11.8% was achieved using TiO2 nanorods with water–HCl solution, which is the highest among the reported TiO2 nanorod based cells.

 Please wait while we load your content...
Please wait while we load your content...