MgH2–TiH2 mixture as an anode for lithium-ion batteries: synergic enhancement of the conversion electrode electrochemical performance†
Abstract
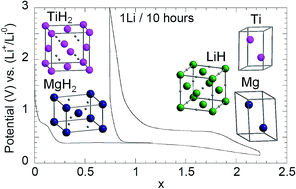
A 0.7MgH2 + 0.3TiH2 mixture was prepared by reactive grinding of Mg and Ti powders under hydrogen and tested as a conversion electrode for lithium-ion batteries. This composite presents superior electrochemical properties compared to MgH2 or TiH2 based electrodes, either in terms of reversible capacities or polarization versus applied current rate. A substantial reversible capacity of 1540 mA h g−1 is measured at a suitable potential of 0.52 V vs. Li+/Li0 (current rate 0.1Li h−1). For the same current rate, electrode polarization is limited to 0.2 V and stabilizes at 0.46 V for 1.0Li h−1 while a continuous polarization increase is observed for MgH2 based electrodes. In situ XRD analyses of this composite demonstrate that Mg and Ti hydrides are both electrochemically active and contribute together to the capacity. TiH2 improves MgH2 conversion process kinetics while MgH2 enables a reversible conversion reaction for TiH2. The three consecutive reactions are: (1) MgH2 + 2Li+ + 2e− ⇌ Mg + 2LiH; (2) δ-TiH2 + xLi+ + xe− ⇌ δ-TiH2−x + xLiH (x ≤ 0.5) and (3) δ-TiH2−x + (2 − x)Li+ + (2 − x)e− ⇌ α-Ti + (2 − x)LiH (x = 0.5). This is, to our knowledge, the first example of a reversible lithium conversion process with TiH2 powder.

 Please wait while we load your content...
Please wait while we load your content...