Carbon dots modified mesoporous organosilica as an adsorbent for the removal of 2,4-dichlorophenol and heavy metal ions†
Abstract
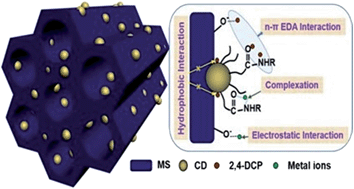
Periodic mesoporous organosilica embedded with carbon dots are adopted as the adsorbent for removal of the toxic organic pollutant 2,4-dichlorophenol and inorganic metal ions Hg(II), Cu(II), and Pb(II). The composite possesses an ordered 2D hexagonal mesostructure with a space group of p6mm, high specific surface area (∼468.46 m2 g−1), and uniform pore size (∼5.50 nm). The surface is covered by about 1–2 layers of carbon dot nanoparticles. The maximum adsorption capacity for 2,4-dichlorophenol is 99.70 mg g−1, and the distribution coefficient of metal ions between adsorbent and solution phases is in the range of 2.60–7.41, following the order of Hg(II) > Cu(II) > Pb(II). The Cu(II) and Pb(II) adsorption stays nearly fixed while Hg(II) adsorption is depressed by ∼45% in a mixed solution of metal ions. The Cu(II) and Hg(II) adsorption shows unapparent variation but Pb(II) adsorption is improved by ∼55% in a mixed solution of metal ion and 2,4-dichlorophenol. In contrast, all metal ions lead to the depression of 2,4-dichlorophenol adsorption by 37% (Pb(II)), 45% (Cu(II)), and 48% (Hg(II)). Finally, the n–π electron donor–acceptor interaction between O- and N-containing groups in mesoporous organosilica and the benzene ring in 2,4-dichlorophenol is revealed to be responsible for the enhanced adsorption of 2,4-dichlorophenol, while the electrostatic force and complex formation between metal ions and amide groups co-contribute to the improvement of metal ions adsorption.

 Please wait while we load your content...
Please wait while we load your content...