Morphological changes and electrochemical response of mixed nickel manganese oxides as charge storage electrodes
Abstract
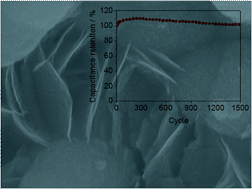
Nickel manganese (Ni–Mn) oxide films were prepared by potentiostatic electrodeposition over stainless steel and post-thermal annealing at 250 °C. Morphological and structural changes were observed depending on the Ni to Mn ratio in the electrolyte. Single-phase Ni1−xMnxO oxide was formed at low Mn content, whereas two phases composed of Ni1−xMnxO and NixMn3−xO4 were formed at high Mn content. Electrochemical studies revealed an increase of the specific capacitance of the mixed Ni–Mn oxide electrodes compared to the single metal oxides, thus pin-pointing a synergistic effect. The Ni–Mn oxide film displayed a specific capacitance of about 300 F g−1 in a potential window of 0.5 V at an applied current density of 1 A g−1. The rate capability of the oxides was 58% when the applied current was increased from 1 A g−1 to 10 A g−1. The Ni–Mn oxide film exhibits excellent cycling stability with 100% capacitance retention after 1500 cycles.

 Please wait while we load your content...
Please wait while we load your content...