Synthesis of hexagonal MoO3 nanorods and a study of their electrochemical performance as anode materials for lithium-ion batteries†
Abstract
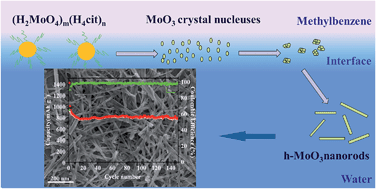
Hexagonal MoO3 nanorods with an average diameter of 40 nm have been synthesized in an immiscible mixture of water and methylbenzene. Both citric acid, which can chelate molybdic acid in water solution, and the interface reaction occurring between the two phases of the mixture are favorable for the formation of hexagonal MoO3 nanorods. As an anode material for lithium-ion batteries, hexagonal MoO3 nanorods exhibit a capacity of over 780 mA h g−1 after 150 cycles at 150 mA g−1, which is higher than that of hexagonal MoO3 microrods with diameters of 2–3 μm.

 Please wait while we load your content...
Please wait while we load your content...