Effects of Mg doping on the remarkably enhanced electrochemical performance of Na3V2(PO4)3 cathode materials for sodium ion batteries†
Abstract
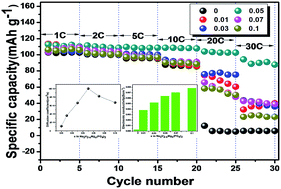
Na3V2−xMgx(PO4)3/C composites with different Mg2+ doping contents (x = 0, 0.01, 0.03, 0.05, 0.07 and 0.1) were prepared by a facile sol–gel method. The doping effects on the crystal structure were investigated by XRD, XPS and EXAFS. The results show that low dose doping of Mg2+ does not alter the structure of the material, and magnesium is successfully substituted for the vanadium site. The Mg doped Na3V2−xMgx(PO4)3/C composites exhibit significant improvements on the electrochemical performance in terms of the rate capability and cycle performance, especially for the Na3V1.95Mg0.05(PO4)3/C. For example, when the current density increased from 1 C to 30 C, the specific capacity only decreased from 112.5 mA h g−1 to 94.2 mA h g−1 showing very good rate capability. Moreover, even cycling at a high rate of 20 C, an excellent capacity retention of 81% is maintained from the initial value of 106.4 mA h g−1 to 86.2 mA h g−1 at the 50th cycle. Enhanced rate capability and cycle performance can be attributed to the optimized particle size, structural stability and enhanced ionic and electronic conductivity induced by Mg doping.


 Please wait while we load your content...
Please wait while we load your content...