Single-ion dominantly conducting polyborates towards high performance electrolytes in lithium batteries†
Abstract
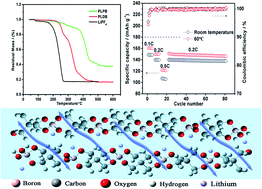
A couple of thermally stable polyborate salts, polymeric lithium pentaerythrite borate (PLPB) and polymeric lithium di(trimethylolpropane)borate (PLDB), for applications in lithium ion batteries were synthesized via a facile one-step reaction in aqueous solution. Both the lithium polyborate salts exhibited a high thermal decomposition temperature at about 240 °C. Besides, their corresponding single-ion dominantly conducting gel polymer electrolytes of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1 : 1, v/v) swollen PLPB@PVDF-HFP (poly(vinylidenefluoride-co-hexafluoropropene)) and PLDB@PVDF-HFP exhibited favorable ionic conductivity over a wide temperature range, superior electrochemical stability, high lithium ion transference number and Al passivating ability. The Li/LiFePO4 batteries using these single-ion dominantly conducting electrolytes exhibited stable charge–discharge behavior and excellent cycling performance both at room temperature and at elevated temperatures. These superior performances could make this class of gel polymer electrolytes very promising candidates for lithium batteries especially at elevated temperatures.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...