Palladium nanoparticles in catalytic carbon nanoreactors: the effect of confinement on Suzuki–Miyaura reactions†
Abstract
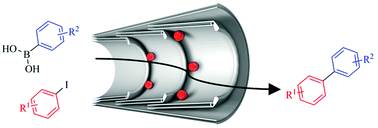
We explore the construction and performance of a range of catalytic nanoreactors based on palladium nanoparticles encapsulated in hollow graphitised nanofibres. The optimum catalytic material, with small palladium nanoparticles located almost exclusively at the graphitic step-edges within nanoreactors, exhibits attractive catalytic properties in Suzuki–Miyaura cross-coupling reactions. Confinement of nanoparticles at the step-edges facilitates retention of catalytic centres and recycling of catalytic nanoreactors without any significant loss of activity or selectivity over multiple catalytic cycles. Furthermore, careful comparison of the catalytic properties of palladium nanoparticles either on or in nanoreactors reveals that nanoscale confinement of catalysts fundamentally affects the pathways of the Suzuki–Miyaura reaction, with the yield and selectivity for the cross-coupled product critically dependent on the steric properties of the aryl iodide reactant, whereas no effects of confinement are observed for aryl boronic acid reactants possessing substituents in different positions. These results indicate that the oxidative addition step of the Suzuki–Miyaura reaction occurs at the step-edge of nanofibres, where the mechanisms and kinetics of chemical reactions are known to be sensitive to nanoscale confinement, and thus the extent of confinement in carbon nanoreactors can be discretely controlled by careful selection of the aryl iodide reactant.

 Please wait while we load your content...
Please wait while we load your content...