Effect of spray-drying and cryo-milling on the CO2 absorption performance of C60 cross-linked polyethyleneimine†
Abstract
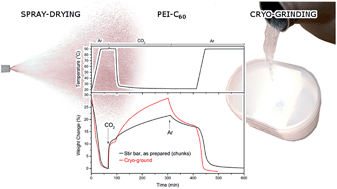
The high CO2 capacity of PEI-C60 conjugates is impeded by a slow rate of absorption. A limiting factor to this rate is proposed to be the surface area available for the rapid contact between amine functional groups of PEI and CO2. Increasing the surface area by spray-drying a solution of reagents is proposed as a route to larger surface area products. In this work we investigate process changes to control absorption chemistry. Reagent solutions were spray-dried in different experimental conditions of concentration, drying temperature, and feed pressure. The results indicate that the rate of CO2 absorption at room temperature can be improved by a factor of 2.5 times by spray drying the product when compared to the product obtained using sonication. Given the rubbery nature of PEI-C60 the surface area, and hence CO2 capacity, could be increased using cryogenic grinding in liquid nitrogen; however, the results show that this has limited effect on the surface area of the absorbent prepared using sonication. Only compared to the hard chunks obtained via stir bar synthesis was the surface area doubled, in contrast to the rubbery product obtained using ultrasonication the area did not change significantly. Interestingly, doubling the surface area, the rate of absorption of wet CO2 at high temperature did not change, while that at low temperature doubled in rate, consistent with the presence of diffusion limitations manly at low temperature.

 Please wait while we load your content...
Please wait while we load your content...