Effect of carbon content on the structure and electronic properties of silicon oxycarbide anodes for lithium-ion batteries: a first-principles study
Abstract
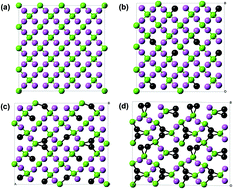
The silicon oxycarbide (SiCO) anode shows good electrochemical performance with regards to Li insertion and shows a three times larger lithium capacity than does graphite. Although published experiments have provided effective compositions of SiCO for Li anode materials, there is little understanding of the atomic details and bonding mechanism of the lithiation process. In this work, first-principles calculations were used to investigate the behavior of lithiation of SiCO with different carbon contents. Based on the lithiated structures and radial distribution functions, Li atoms prefer to bond with oxygen atoms, and prefer to be accommodated near the free carbon structure in SiCO. This result indicates that the SiCO, with its relatively high carbon content, has a relatively large lithium capacity, which is also indicated by the results of energy of formation calculations. The reduced volume upon lithium insertion reflects the formation of new Li compounds, and the compounds that have denser structures are those with higher C contents. The calculations provide an insight into the mechanism of the lithiation of SiCO anode materials for Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...