In situ high-energy synchrotron X-ray diffraction studies and first principles modeling of α-MnO2 electrodes in Li–O2 and Li-ion coin cells†
Abstract
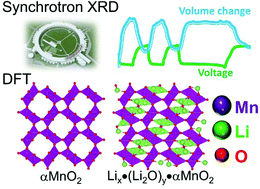
Despite their technological challenges, non-aqueous rechargeable lithium–oxygen cells offer extremely high theoretical energy densities and are therefore attracting much attention in a rapidly emerging area of electrochemical research. Early results have suggested that, among the transition metal oxides, alpha manganese dioxide (α-MnO2) appears to offer electrocatalytic properties that can enhance the electrochemical properties of Li–O2 cells, particularly during the early cycles. In this study, we have investigated the hybrid Li-ion/Li–O2 character of α-MnO2 electrodes in Li–O2 coin cells by in situ high-energy synchrotron X-ray diffraction, and compared the results with conventional Li/α-MnO2 coin cells assembled under argon. Complementary first principles density functional theory calculations have been used to shed light on competing lithium insertion and lithium and oxygen insertion reactions within the α-MnO2 tunnel structure during discharge, relative to lithium peroxide or lithium oxide formation.

 Please wait while we load your content...
Please wait while we load your content...