Thermodynamic complexity of carbon capture in alkylamine-functionalized metal–organic frameworks†
Abstract
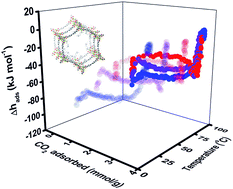
For coordinatively unsaturated metal–organic frameworks (MOFs), the metal centers can be functionalized as CO2 capture/storage adsorbents by grafting species having specific active groups. We report direct measurement of enthalpy of adsorption of CO2 on an alkylamine-appended MOF, mmen-Mg2(dobpdc) employing gas adsorption calorimetry at 298, 323 and 348 K. This methodology provides, for the first time, the detailed dependence of energy and entropy of sorption as a function of coverage and temperature. The enthalpy data suggest three types of adsorption events: strongest exothermic initial chemisorption at low coverage, majority moderate chemisorption at intermediate loading and weakest physisorption at highest coverage. The partial molar properties and isotherms are consistent with the presence of two different potential chemisorption mechanisms: 2 : 1 (amine–CO2) stoichiometry near zero coverage and 1 : 1 afterwards. Both chemical potential and differential enthalpy of adsorption become less negative with increasing temperature, implying increasing adsorbent entropy at elevated temperature. These observations are consistent with weaker CO2 binding at higher temperature.

 Please wait while we load your content...
Please wait while we load your content...