Carbon-coated α-Fe2O3 nanostructures for efficient anode of Li-ion battery†
Abstract
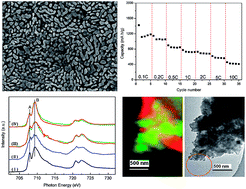
Carbon-coated α-Fe2O3 nanostructures, as the anode of Li-ion battery, have been deposited on the stainless steel substrate by a facile pyrolysis of ferrocene. The anode shows a high reversible capacity of 1138 mA h g−1 after 300 cycles at the current density of 500 mA g−1 and maintains a good capacity of 458.8 mA h g−1 even when cycled at the high current density of 10 000 mA g−1. This high capacity can be associated to the nanostructure and the carbon layer coated on hematite. Moreover, the mechanism for the capacity evolution with cycling has been investigated by scanning transmission X-ray microscopy (STXM). The results reveal that the detailed composition and electronic structure change in the cycling process. Fe chemical state plays a critical role in the capacity evolution and a low oxidation state of Fe (such as Fe2+) might reduce the capacity by trapping Li+ ions, and the recovery of Fe2+ to hematite (Fe3+) significantly enhances the capacity. Data also show the growth and inhomogeneous distribution of a solid electrolyte interphase (SEI) layer containing carbon-based film, Li2O and Li2CO3. The facile synthesis of carbon-coated α-Fe2O3 opens an efficient way for large-scale anode production of Li-ion batteries, and the STXM study provides new insights into the mechanism of hematite-based Li-ion battery.

 Please wait while we load your content...
Please wait while we load your content...