Covalently crosslinked graphene oxide membranes by esterification reactions for ions separation
Abstract
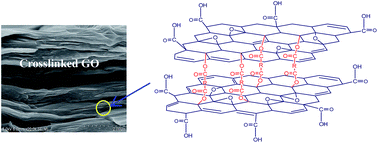
We present a method to prepare covalently crosslinked graphene oxide (GO) membranes with adjustable intersheet spacing by esterification reactions, using dicarboxylic acids, diols or polyols as the crosslinker and hydrochloric acid as the catalyst. For dicarboxylic acids, with the increased length of the molecular chain, the intersheet spacing, the elastic moduli and permeation fluxes of GO membranes generally increases. The elastic modulus of the hexanedioic acid crosslinked membrane is 15.6 times as that of pristine, and the selectivity of K+/Mg2+ attains to 6.1. There exists an optimum chain length of crosslinkers. For diols or polyols, the hydrophobic substituents (–CH3) tend to enlarge the intersheet spacing of GO membranes, while the hydrophilic substituents (–OH) favor the penetration of hydrated ions. The elastic moduli, permeation fluxes and selectivity of diols or polyols crosslinked membranes are relatively lower than those of dicarboxylic acids crosslinked ones.

 Please wait while we load your content...
Please wait while we load your content...