In situ spectroscopy studies of CO2 adsorption in a dually functionalized microporous metal–organic framework†
Abstract
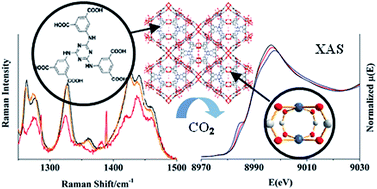
The activation and CO2 gas adsorption processes in the rht-type metal-organic framework, [Cu3(TDPAT) (H2O)3]·10H2O·5DMA (TDPAT = 2,4,6-tris(3,5-dicarboxylphenylamino)-1,3,5-triazine) were investigated on the molecular level using several spectroscopic characterization methods. The remarkable selectivity of this framework for CO2 is attributed to the high density of coordinatively unsaturated metal nodes and amine linker functionality that both serve as gas binding sites. Spectroscopic evidence for these binding interactions as well as the concomitant electronic and molecular level structure changes of the host framework and CO2 guest is derived from a combination of in situ UV-vis diffuse reflectance, X-ray absorption and Raman spectroscopy studies. Results showed that the activation process produced subtle structural rearrangements of the framework that may be influencing the binding interactions upon subsequent CO2 adsorption.

 Please wait while we load your content...
Please wait while we load your content...