Cathodic corrosion: an electrochemical approach to capture Zintl compounds for powder materials†
Abstract
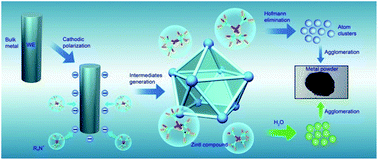
The chemical procedure to obtain the Zintl polyanions is considerably tedious due to their own instability. We have examined cathodic corrosion for all the five group 14 elements, and polyatomic group 14 Zintl intermediates (R4N+)4M94− (M = Sn, Pb) have been electrochemically captured through cathodic corrosion, proved by in situ Raman spectroscopy. Moreover, metal powder materials are obtained through their simultaneous and/or subsequent Hofmann elimination or oxidation by water at room temperature. It is the strong covalent bonds and cubic face-centered diamond structures that determine the chemical stability of the diamond, silicon and germanium, while the metallic bonds of tin and lead make the cathodic generation of Zintl intermediates (R4N+)4M94− feasible. This work opens up new strategies to design powder materials for scientific research and industrial applications.

 Please wait while we load your content...
Please wait while we load your content...