Task-specific functionalization of graphene for use as a cathode catalyst support for carbon dioxide conversion†
Abstract
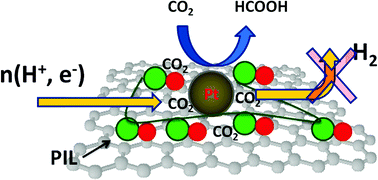
This study describes the potential advantages of task-specific functionalization of graphene for carbon dioxide capture and conversion. An ionic liquid was employed as a functional moiety on a graphene catalyst support since it shows reversible interactions with CO2 molecules. In this study, graphene was synthesized by a hydrogen-assisted low-temperature-exfoliation method and functionalized using a polymerized ionic liquid (PIL). Adsorption analysis shows that PIL functionalization improves the CO2 adsorption capacity of graphene significantly. Catalytic nanoparticles were dispersed over the catalyst supports by a microwave assisted polyol reduction method and the catalysts were employed as the cathode catalyst in a polymer electrolyte membrane (PEM) CO2 conversion cell. The cell hydrogenates CO2 into formic acid at the cathode, which was quantified by spectrophotometry. The PIL functionalized support shows a higher formic acid formation rate compared to a pure support under similar experimental conditions since PIL functionalization improves the interactions between the catalyst support and the CO2 molecules. The cells were tested with discontinuous and continuous CO2 supplies.

 Please wait while we load your content...
Please wait while we load your content...