Alzheimer's peptide amyloid-β, fragment 22–40, perturbs lipid dynamics†
Abstract
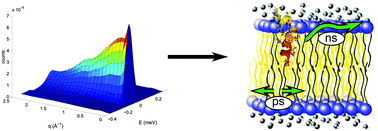
The peptide amyloid-β (Aβ) interacts with membranes of cells in the human brain and is associated with Alzheimer's disease (AD). The intercalation of Aβ in membranes alters membrane properties, including the structure and lipid dynamics. Any change in the membrane lipid dynamics will affect essential membrane processes, such as energy conversion, signal transduction and amyloid precursor protein (APP) processing, and may result in the observed neurotoxicity associated with the disease. The influence of this peptide on membrane dynamics was studied with quasi-elastic neutron scattering, a technique which allows a wide range of observation times from picoseconds to nanoseconds, over nanometer length scales. The effect of the membrane integral neurotoxic peptide amyloid-β, residues 22–40, on the in- and out-of-plane lipid dynamics was observed in an oriented DMPC/DMPS bilayer at 15 °C, in its gel phase, and at 30 °C, near the phase transition temperature of the lipids. Near the phase-transition temperature, a 1.5 mol% of peptide causes up to a twofold decrease in the lipid diffusion coefficients. In the gel-phase, this effect is reversed, with amyloid-β(22–40) increasing the lipid diffusion coefficients. The observed changes in lipid diffusion are relevant to protein–protein interactions, which are strongly influenced by the diffusion of membrane components. The effect of the amyloid-β peptide fragment on the diffusion of membrane lipids will provide insight into the membrane's role in AD.

 Please wait while we load your content...
Please wait while we load your content...