Self-assembly and morphological transitions of random amphiphilic poly(β-d-glucose-co-1-octyl) phosphazenes†
Abstract
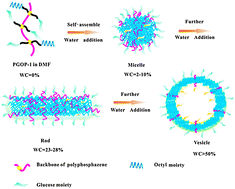
The amphiphilic random copolymer poly(β-D-glucose-co-1-octyl)phosphazene (PGOP) can undergo continuous morphological transitions in DMF–water mixed solvents. In this study, the ratio of glucose moieties to octyl moieties was controlled via a two-step thiol–ene reaction. As a result, polyphosphazenes with glycosyl functionalization degrees of 58.1% (PGOP-1), 74.1% (PGOP-2) and 87.0% (PGOP-3) were obtained. These amphiphilic polyphosphazenes self-assemble in both water and water–DMF mixtures. Several self-assembled morphologies including spheres, rods and vesicles were formed though careful control of the water content (WC) in the DMF solvent as well as of the hydrophilicity or hydrophobicity of the copolymers. We also found that an increase in the hydrophobic proportion led to faster morphological transitions at a constant WC. The thermodynamics of micellization were also studied by Isothermal Titration Calorimetry (ITC), and the strong hydrophobic interactions in PGOP-1 were demonstrated by their highly exothermic nature. These self-assemblies have potential applications in biosensing, lectin adsorption and drug loading with controlled release.

 Please wait while we load your content...
Please wait while we load your content...