Preparation, characterization and magnetic behavior of a spin-labelled physical hydrogel containing a chiral cyclic nitroxide radical unit fixed inside the gelator molecule†
Abstract
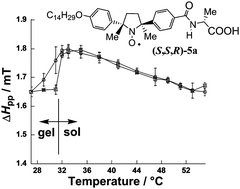
An optically active amphiphilic nitroxide radical compound [(S,S,R)-5a], which contains a paramagnetic (2S,5S)-2,5-dimethyl-2,5-diphenylpyrrolidine-N-oxyl radical group fixed in the inner position together with a hydrophobic long alkyl chain and a hydrophilic (R)-alanine residue in the opposite terminal positions, was found to serve as a low-molecular-weight gelator in H2O to give rise to a spin-labelled physical hydrogel. Characterization of the hydrogel was performed by microscopic (SEM, TEM and AFM) techniques, XRD and SAXS measurements, and IR, UV and CD spectroscopies. The gel–sol transition temperature was determined by EPR spectral line-width (ΔHpp) analysis. Measurement of the temperature dependence of relative paramagnetic susceptibility (χrel) for the hydrogel and sol phases was achieved by means of the double-integration of VT-EPR spectra.

 Please wait while we load your content...
Please wait while we load your content...