Mixed mode of dissolving immersed nanodroplets at a solid–water interface†
Abstract
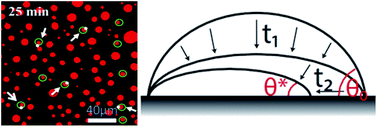
The dissolution dynamics of microscopic oil droplets (less than 1 μm in height, i.e. nanodroplets) on a hydrophobilized silicon surface in water was experimentally studied. The lateral diameter was monitored using confocal microscopy, whereas the contact angle was measured by (disruptive) droplet polymerisation of the droplet. In general, we observed the droplets to dissolve in a mixed mode, i.e., neither in the constant contact angle mode nor in the constant contact radius mode. This means that both the lateral diameter and the contact angle of the nanodroplets decrease during the dissolution process. On average, the dissolution rate is faster for droplets with larger initial size. Droplets with the same initial size can, however, possess different dissolution rates. We ascribe the non-universal dissolution rates to chemical and geometric surface heterogeneities (that lead to contact line pinning) and cooperative effects from the mass exchange among neighbouring droplets.

 Please wait while we load your content...
Please wait while we load your content...