Using the gravitational energy of water to generate power by separation of charge at interfaces†
Abstract
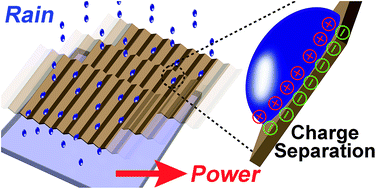
When a fluid comes into contact with a solid surface, charge separates at the interface. This study describes a method that harvests the gravitational energy of water—available in abundance naturally, such as in rain and rivers—through the separation of charge at the interface. Essentially, it is found that water can be charged by flowing it across a solid surface under its own weight; thus, a continuous flow of water can produce a constant supply of power. After optimizing the system, a power of up to ∼170 μW (per Teflon tube of 2 mm in diameter) can be generated. The efficiency, defined as the energy generated by the system over the gravitational energy that the water losses, can reach up to ∼3–4%. In order to generate a continuous stream of positively-charged water, there should also be a constant production of negatively-charged species in the system. Experimental results suggest that the negative charge transfers constantly to the atmosphere due to dielectric breakdown of air. With regards to applications related to high electrical potential of water droplets, the amount of charge generated in a single water droplet is found to be equivalent to that produced by charging the water droplet with a high-voltage power supply operated at ∼5 kV. In general, the energy generated is clean, renewable, and technically simple and inexpensive to produce.
- This article is part of the themed collection: Global Energy Challenges: Biofuels

 Please wait while we load your content...
Please wait while we load your content...