Oxygen deficient α-Fe2O3 photoelectrodes: a balance between enhanced electrical properties and trap-mediated losses†
Abstract
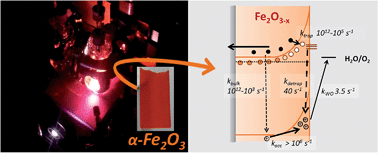
Intrinsic doping of hematite through the inclusion of oxygen vacancies (VO) is being increasingly explored as a simple, low temperature route to preparing active water splitting α-Fe2O3−x photoelectrodes. Whilst it is widely accepted that the introduction of VO leads to improved conductivities, little else is verified regarding the actual mechanism of enhancement. Here we employ transient absorption (TA) spectroscopy to build a comprehensive kinetic model for water oxidation on α-Fe2O3−x. In contrast to previous suggestions, the primary effect of introducing VO is to block very slow (ms) surface hole – bulk electron recombination pathways. In light of our mechanistic research we are also able to identify and address a cause of the high photocurrent onset potential, a common issue with this class of electrodes. Atomic layer deposition (ALD) of Al2O3 is found to be particularly effective with α-Fe2O3−x, leading to the photocurrent onset potential shifting by ca. 200 mV. Significantly TA measurements on these ALD passivated electrodes also provide important insights into the role of passivating layers, that are relevant to the wider development of α-Fe2O3 photoelectrodes.
- This article is part of the themed collections: In celebration of Kazunari Domen’s 65th birthday, 2018 and Global Energy Challenges: Solar Energy

 Please wait while we load your content...
Please wait while we load your content...