Sulfonyl fluorides as privileged warheads in chemical biology
Abstract
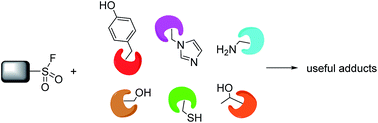
Sulfonyl fluoride electrophiles have found significant utility as reactive probes in chemical biology and molecular pharmacology. As warheads they possess the right balance of biocompatibility (including aqueous stability) and protein reactivity. Their functionality is privileged in this regard as they are known to modify not only reactive serines (resulting in their common use as protease inhibitors), but also context-specific threonine, lysine, tyrosine, cysteine and histidine residues. This review describes the application of sulfonyl fluoride probes across various areas of research and explores new approaches that could further enhance the chemical biology toolkit. We believe that sulfonyl fluoride probes will find greater utility in areas such as covalent enzyme inhibition, target identification and validation, and the mapping of enzyme binding sites, substrates and protein–protein interactions.
- This article is part of the themed collection: Global challenges: Health & Food

 Please wait while we load your content...
Please wait while we load your content...