Two-coordinate group 14 element(ii) hydrides as reagents for the facile, and sometimes reversible, hydrogermylation/hydrostannylation of unactivated alkenes and alkynes†
Abstract
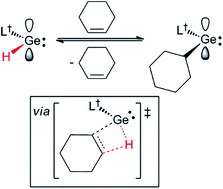
Reactions of the solution stable, two-coordinate hydrido-tetrylenes, :E(H)(L†) (E = Ge or Sn; L† = –N(Ar†)(SiPri3); Ar† = C6H2{C(H)Ph2}2Pri-2,6,4), with a variety of unactivated cyclic and acyclic alkenes, and one internal alkyne, lead to the rapid and regiospecific hydrometallation of the unsaturated substrate at ambient temperature. The products of the reactions, [L†E(C2H4R)] (E = Ge or Sn, R = H, Ph or But), [L†E{CH(CH2)3(CH2)n}] (E = Ge, n = 1, 2 or 3; E = Sn, n = 1) and [L†E{C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)(Me)}], include the first structurally characterised examples of two-coordinate amido/alkyl germylenes and stannylenes. The cycloalkene hydrometallation reactions are cleanly reversible under ambient conditions, a process which computational and experimental van't Hoff analyses suggest proceeds via β-hydride elimination from the metal coordinated cycloalkyl ligand. Similarly, the reactions of :Ge(H)(L†) with 1,5-cyclooctadiene and 2-methyl-2-butene, both likely proceed via β-hydride elimination processes, leading to the clean isomerisation of the alkene involved, and its subsequent hydrogermylation, to give [L†Ge(2-cyclooctenyl)] and [L†Ge{C2H4C(H)Me2}], respectively. Reactions of [L†GeEt] and [L†Ge(C5H9)] with the protic reagents, HCl, NH3 and EtOH, lead to oxidative addition to the germanium(II) centre, and formation of the stable chiral germanium(IV) complexes, [L†Ge(C5H9)(H)Cl] and [L†Ge(Et)(H)R] (R = NH2 or OEt). In contrast, related reactions between [L†SnEt] and ButOH or TEMPOH (TEMP = 2,2,6,6-tetramethylpiperidinyl) proceed via ethane elimination, affording the tin(II) products, [L†SnR] (R = OBut or OTEMP). In addition, the oxidation of [L†Ge(C6H11)] and [L†Sn(C2H4But)] with O2 yields the oxo-bridged metal(IV) dimers, [{L†(C6H11)Ge(μ-O)}2] and [{L†(ButC2H4)Sn(μ-O)}2], respectively.
C(H)(Me)}], include the first structurally characterised examples of two-coordinate amido/alkyl germylenes and stannylenes. The cycloalkene hydrometallation reactions are cleanly reversible under ambient conditions, a process which computational and experimental van't Hoff analyses suggest proceeds via β-hydride elimination from the metal coordinated cycloalkyl ligand. Similarly, the reactions of :Ge(H)(L†) with 1,5-cyclooctadiene and 2-methyl-2-butene, both likely proceed via β-hydride elimination processes, leading to the clean isomerisation of the alkene involved, and its subsequent hydrogermylation, to give [L†Ge(2-cyclooctenyl)] and [L†Ge{C2H4C(H)Me2}], respectively. Reactions of [L†GeEt] and [L†Ge(C5H9)] with the protic reagents, HCl, NH3 and EtOH, lead to oxidative addition to the germanium(II) centre, and formation of the stable chiral germanium(IV) complexes, [L†Ge(C5H9)(H)Cl] and [L†Ge(Et)(H)R] (R = NH2 or OEt). In contrast, related reactions between [L†SnEt] and ButOH or TEMPOH (TEMP = 2,2,6,6-tetramethylpiperidinyl) proceed via ethane elimination, affording the tin(II) products, [L†SnR] (R = OBut or OTEMP). In addition, the oxidation of [L†Ge(C6H11)] and [L†Sn(C2H4But)] with O2 yields the oxo-bridged metal(IV) dimers, [{L†(C6H11)Ge(μ-O)}2] and [{L†(ButC2H4)Sn(μ-O)}2], respectively.
- This article is part of the themed collection: RACI100: Celebrating Australian Chemistry

 Please wait while we load your content...
Please wait while we load your content...