A highly diastereoselective “super silyl” governed aldol reaction: synthesis of α,β-dioxyaldehydes and 1,2,3-triols†
Abstract
A highly diastereoselective approach for the synthesis of protected α,β-dioxyaldehydes derived from (Z)-tris(trimethylsilyl)silyl “super silyl” enol ethers is described. A general and highly syn-stereoselective aldol reaction directed by the “super silyl” group catalyzed by triflimide (HNTf2) is developed providing α,β-dioxyaldehydes and 1,2,3-triol fragments which can be a useful platform for the elaboration of natural and unnatural sugar derivatives.
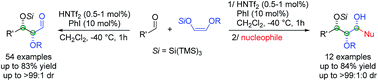


 Please wait while we load your content...
Please wait while we load your content...