C–H arylation and alkenylation of imidazoles by nickel catalysis: solvent-accelerated imidazole C–H activation†
Abstract
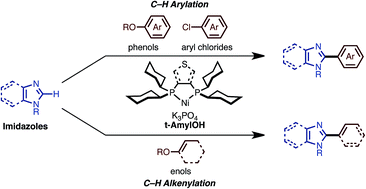
The first nickel-catalyzed C–H arylations and alkenylations of imidazoles with phenol and enol derivatives are described. Under the influence of Ni(OTf)2/dcype/K3PO4 (dcype: 1,2-bis(dicyclohexylphosphino)ethane) in t-amyl alcohol, imidazoles can undergo C–H arylation with phenol derivatives. The C–H arylation of imidazoles with chloroarenes as well as that of thiazoles and oxazoles with phenol derivatives can also be achieved with this catalytic system. By changing the ligand to dcypt (3,4-bis(dicyclohexylphosphino)thiophene), enol derivatives could also be employed as coupling partners achieving the C–H alkenylation of imidazoles as well as thiazoles and oxazoles. Thus, a range of C2-arylated and alkenylated azoles can be synthesized using this newly developed nickel-based catalytic system. The key to the success of the C–H coupling of imidazoles is the use of a tertiary alcohol as solvent. This also allows the use of an air-stable nickel(II) salt as the catalyst precursor.

 Please wait while we load your content...
Please wait while we load your content...