Enantioselective palladium(0)-catalyzed intramolecular cyclopropane functionalization: access to dihydroquinolones, dihydroisoquinolones and the BMS-791325 ring system†
Abstract
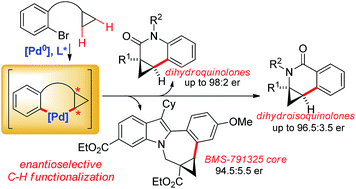
Taddol-based phosphoramidite ligands enable enantioselective palladium(0)-catalyzed C–H arylation of cyclopropanes. The cyclized products are obtained in high yields and enantioselectivities. The reported method provides efficient access to a broad range of synthetically attractive cyclopropyl containing dihydroquinolones and dihydroisoquinolones as well as allows for an efficient enantioselective construction of the 7-membered ring of the cyclopropyl indolobenzazepine core of BMS-791325.

 Please wait while we load your content...
Please wait while we load your content...