Liquid-crystalline calcium carbonate: biomimetic synthesis and alignment of nanorod calcite†
Abstract
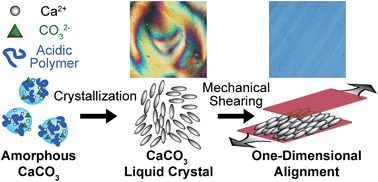
Liquid-crystalline CaCO3 has been prepared for the first time. The nanorods of CaCO3 calcite are obtained by bio-inspired crystallization through aqueous colloidal precursors of amorphous CaCO3 stabilized by poly(acrylic acid). The synthesized calcite nanocrystals have well-tuned morphologies that are preferable for formation of liquid-crystalline phases in concentrated aqueous colloidal solution. The one-dimensional alignment of calcite crystals is achieved by mechanical shearing of the aqueous colloidal solution showing liquid-crystalline phases. These CaCO3-based liquid crystals formed by a self-organization process in mild conditions may have great potential for use as environmentally friendly materials.

 Please wait while we load your content...
Please wait while we load your content...