Enhancing electron affinity and tuning band gap in donor–acceptor organic semiconductors by benzothiadiazole directed C–H borylation†
Abstract
Electrophilic borylation using BCl3 and benzothiadiazole to direct the C–H functionalisation of an adjacent aromatic unit produces fused boracyclic materials with minimally changed HOMO energy levels but significantly reduced LUMO energy levels. In situ alkylation and arylation at boron using Al(alkyl)3 or Zn(aryl)2 is facile and affords boracycles that possess excellent stability towards protic solvents, including water, and display large bathochromic shifts leading to far red/NIR emission in the solid state with quantum yields of up to 34%. Solution fabricated OLEDs with far red/NIR electroluminescence are reported with EQEs > 0.4%.
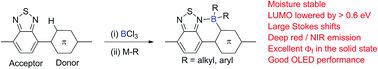

 Please wait while we load your content...
Please wait while we load your content...