Conformational changes in amyloid-beta (12–28) alloforms studied using action-FRET, IMS and molecular dynamics simulations†
Abstract
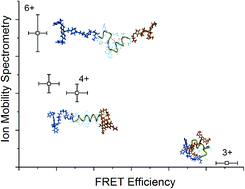
Small oligomers of the amyloid beta protein (Aβ) have been implicated as the neurotoxic agent leading to Alzheimer's disease, and in particular mutations in the hydrophobic core region comprising amino acids L17 to A21 have a large influence on the propensity for aggregate formation. It has been shown that the F19P alloform of Aβ forms small aggregates, but does not proceed to form large fibrils and plaques. In order to understand the origin of this behavior, the gas phase conformations for the different charge states of the wild-type 12–28 fragment of the amyloid beta and its F19P alloform were studied by a combination of action-FRET, ion-mobility spectrometry (IMS) and molecular dynamics simulations. Comparison of the experimental and theoretical action-FRET efficiencies and collision cross sections allowed the determination of the lowest energy conformational family for each alloform and charge state. For both alloforms, it was found that there is a change from globular to helical structure between the 3+ and 4+ charge states. Additional protonation to give 5+ and 6+ charge states caused unfolding of this helical motif, with the wild alloform showing β-turn like motifs and the F19P alloform random coil motifs. The presence of the helical to β-turn structural transition in the wild, but not the F19P, alloform may help to elucidate the origin of the large difference in aggregation behavior of the two alloforms.

 Please wait while we load your content...
Please wait while we load your content...