Photoanodic and photocathodic behaviour of La5Ti2CuS5O7 electrodes in the water splitting reaction†
Abstract
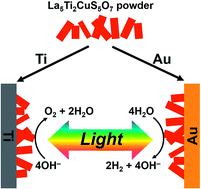
The particulate semiconductor La5Ti2CuS5O7 (LTC) with a band gap energy of 1.9 eV functioned as either a photocathode or a photoanode when embedded onto Au or Ti metal layers, respectively. By applying an LTC/Au photocathode and LTC/Ti photoanode to, respectively, photoelectrochemical (PEC) water reduction and oxidation concurrently, zero-bias overall water splitting was accomplished under visible light irradiation. The band structures of LTC/Au and LTC/Ti calculated using a semiconductor device simulator (AFORS-HET) confirmed the critical role of the solid/solid junction of the metal back contact in the charge separation and PEC properties of LTC photoelectrodes. The prominently long lifetime of photoexcited charge carriers in LTC, confirmed by transient absorption spectroscopy, allowed the utilization of both photoexcited electrons and holes depending on the band structure at the solid/solid junction.
- This article is part of the themed collections: In celebration of Kazunari Domen’s 65th birthday, 2018 and Global Energy Challenges: Hydrogen Energy

 Please wait while we load your content...
Please wait while we load your content...