Carbene catalyzed umpolung of α,β-enals: a reactivity study of diamino dienols vs. azolium enolates, and the characterization of advanced reaction intermediates†
Abstract
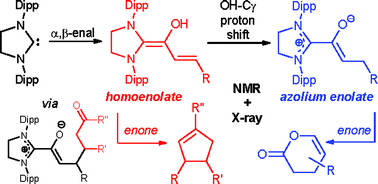
Since their discovery by Bode and Glorius in 2004, N-heterocyclic carbene catalyzed conjugate umpolung reactions of α,β-enals have been postulated to involve the formation of diamino dienols (“homoenolates”) and/or azolium enolates (“enolates”), typically followed by addition to electrophiles, e.g. Michael-acceptors. In this article, we provide evidence, for the first time, for the postulated individual and specific reactivity patterns of diamino dienols (γ-C–C-bond formation) vs. azolium enolates (β-C–C-bond formation). Our study is based on the pre-formation of well defined diamino dienols and azolium enolates, and the in situ NMR monitoring of their reactivities towards enone electrophiles. Additionally, reaction intermediates were isolated and characterized, inter alia by X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...