A prochelator peptide designed to use heterometallic cooperativity to enhance metal ion affinity†
Abstract
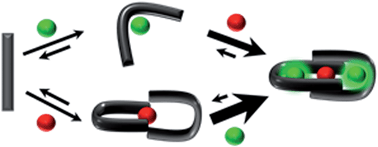
A peptide has been designed so that its chelating affinity for one type of metal ion regulates its affinity for a second, different type of metal ion. The prochelator peptide (PCP), which is a fusion of motifs evocative of calcium loops and zinc fingers, forms a 1 : 2 Zn : peptide complex at pH 7.4 that increases its affinity for Zn2+ ∼3-fold in the presence of Tb3+ (log β2 from 13.8 to 14.3), while the 1 : 1 luminescent complex with Tb3+ is brighter, longer lived, and 20-fold tighter in the presence of Zn2+ (log K from 6.2 to 7.5). This unique example of cooperative, heterometallic allostery in a biologically compatible construct suggests the possibility of designing conditionally active metal-binding agents that could respond to dynamic changes in cellular metal status.

 Please wait while we load your content...
Please wait while we load your content...