Boryl substitution of functionalized aryl-, heteroaryl- and alkenyl halides with silylborane and an alkoxy base: expanded scope and mechanistic studies†
Abstract
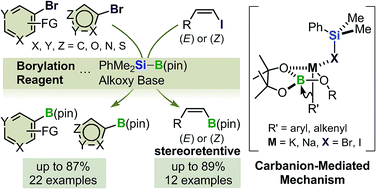
A transition-metal-free method has been developed for the boryl substitution of functionalized aryl-, heteroaryl- and alkenyl halides with a silylborane in the presence of an alkali-metal alkoxide. The base-mediated boryl substitution of organohalides with a silylborane was recently reported to provide the corresponding borylated products in good to high yields, and exhibit good functional group compatibility and high tolerance to steric hindrance. In this study, the scope of this transformation has been extended significantly to include a wide variety of functionalized aryl-, heteroaryl- and alkenyl halides. In particular, the boryl substitution of (E)- and (Z)-alkenyl halides proceeded smoothly to afford the corresponding alkenyl boronates in good to high yields with retention of the configuration using modified reaction conditions. The results of the mechanistic studies suggest that this boryl substitution proceeds via a carbanion-mediated mechanism.

 Please wait while we load your content...
Please wait while we load your content...