Mechanism and selectivity of the dinuclear iron benzoyl-coenzyme A epoxidase BoxB†
Abstract
Benzoyl-CoA epoxidase is a dinuclear iron enzyme that catalyzes the epoxidation reaction of the aromatic ring of benzoyl-CoA with chemo-, regio- and stereo-selectivity. It has been suggested that this enzyme may also catalyze the deoxygenation reaction of epoxide, suggesting a unique bifunctionality among the diiron enzymes. We report a density functional theory study of this enzyme aimed at elucidating its mechanism and the various selectivities. The epoxidation is suggested to start with the binding of the O2 molecule to the diferrous center to generate a diferric peroxide complex, followed by concerted O–O bond cleavage and epoxide formation. Two different pathways have been located, leading to (2S,3R)-epoxy and (2R,3S)-epoxy products, with barriers of 17.6 and 20.4 kcal mol−1, respectively. The barrier difference is 2.8 kcal mol−1, corresponding to a diastereomeric excess of about 99 : 1. Further isomerization from epoxide to phenol is found to have quite a high barrier, which cannot compete with the product release step. After product release into solution, fast epoxide–oxepin isomerization and racemization can take place easily, leading to a racemic mixture of (2S,3R) and (2R,3S) products. The deoxygenation of epoxide to regenerate benzoyl-CoA by a diferrous form of the enzyme proceeds via a stepwise mechanism. The C2–O bond cleavage happens first, coupled with one electron transfer from one iron center to the substrate, to form a radical intermediate, which is followed by the second C3–O bond cleavage. The first step is rate-limiting with a barrier of only 10.8 kcal mol−1. Further experimental studies are encouraged to verify our results.
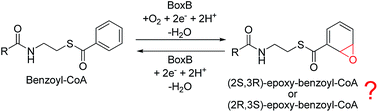

 Please wait while we load your content...
Please wait while we load your content...