Adaptive binding and selection of compressed 1,ω-diammonium-alkanes via molecular encapsulation in water†
Abstract
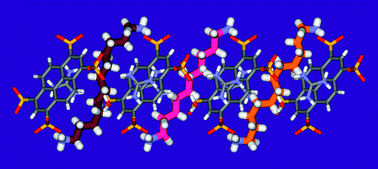
Guest molecules confined inside hollow molecular assemblies and thus protected from their environment can show unexpected structural behavior or special reactivity compared to their behavior in a bulk, unprotected environment. A special case is the coiling behavior of variable-length alkane chains in rigid hydrogen-bonded molecular cages. It has been found before that coiling may occur in such circumstances, but no experimental evidence concerning the exact conformation of the chains has yet been presented. We reveal in this study the self-assembly of a molecular cage in water and the crystalline state from three distinct components in which linear 1,ω-diammonium-alkanes chains are confined with different degrees of compression. The exact coiling behavior is determined from atomic resolution X-ray diffraction showing crenel-like conformations in the compressed state. Chemical selection can be obtained from mixtures of alkane chains via the encapsulation of kinetically stable conformations observed during the encapsulation of pure components. Moreover, it was found that uncompressed and compressed chains can be competitively trapped inside the capsule. These findings may provide insight in areas to a better understanding of biological processes, such as the fatty acid metabolism.

 Please wait while we load your content...
Please wait while we load your content...