Two-photon sensitive protecting groups operating via intramolecular electron transfer: uncaging of GABA and tryptophan†
Abstract
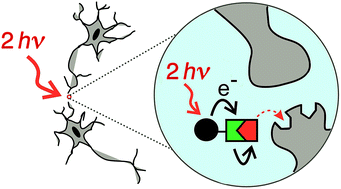
Improved photo-labile protecting groups, with high sensitivity to two-photon excitation, are needed for the controlled release of drugs, as tools in neuroscience and physiology. Here we present a new modular approach to the design of caging groups based on photoinduced electron transfer from an electron-rich two-photon dye to an electron acceptor, followed by scission of an ester to release a carboxylic acid. Three different electron acceptors were tested: nitrobenzyl, phenacyl and pyridinium. The nitrobenzyl system was ineffective, giving only photochemical decomposition and no release of the carboxylic acid. The phenacyl system also performed poorly, liberating the carboxylic acid in 20% chemical yield and 0.2% photochemical yield. The pyridinium system was most successful, and was tested for the release of two carboxylic acids: γ-amino butyric acid (GABA) and tryptophan. The caged GABA undergoes photochemical cleavage with a chemical yield of >95% and a photochemical yield of 1%; it exhibits a two-photon absorption cross section of 1100 GM at 700 nm, corresponding to a two-photon uncaging cross section of 10 ± 3 GM.

 Please wait while we load your content...
Please wait while we load your content...