N-Heterocyclic carbene catalysed redox isomerisation of esters to functionalised benzaldehydes†
Abstract
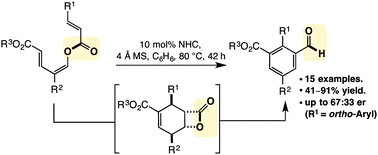
N-Heterocyclic carbene catalysed redox isomerisation with reduction about the carbonyl has been developed in the transformation of trienyl esters to tetrasubstituted benzaldehydes. The reaction proceeds in good to excellent yield, and in cases that provide 2,2′-biaryls, enantioselectivity is observed. Mechanistic studies demonstrate the intermediacy of a cyclohexenyl β-lactone, while implicating formation of the homoenolate as turnover limiting.
- This article is part of the themed collection: RACI100: Celebrating Australian Chemistry

 Please wait while we load your content...
Please wait while we load your content...