On the formation of anions: frequency-, angle-, and time-resolved photoelectron imaging of the menadione radical anion†
Abstract
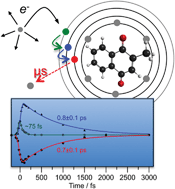
Frequency-, angle-, and time-resolved photoelectron imaging of gas-phase menadione (vitamin K3) radical anions was used to show that quasi-bound resonances of the anion can act as efficient doorway states to produce metastable ground electronic state anions on a sub-picosecond timescale. Several anion resonances have been experimentally observed and identified with the assistance of ab initio calculations, and ground state anion recovery was observed across the first 3 eV above threshold. Time-resolved measurements revealed the mechanism of electronic ground state anion formation, which first involves a cascade of very fast internal conversion processes to a bound electronic state that, in turn, decays by slower internal conversion to the ground state. Autodetachment processes from populated resonances are inefficient compared with electronic relaxation through internal conversion. The mechanistic understanding gained provides insight into the formation of radical anions in biological and astrochemical systems.

 Please wait while we load your content...
Please wait while we load your content...