What can NMR spectroscopy of selenoureas and phosphinidenes teach us about the π-accepting abilities of N-heterocyclic carbenes?†
Abstract
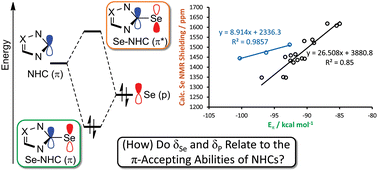
The electronic nature of the interaction of NHCs with metal centres is of interest when exploring their properties, how these properties influence those of metal complexes, and how these properties might depend on ligand structure. Selenourea and phosphinidene complexes have been proposed to allow the measurement of the π-accepting ability of NHCs, independent of their σ-donating ability, via the collection of 77Se or 31P NMR spectra, respectively. Herein, the synthesis and characterisation of selenoureas derived from a range of imidazol-2-ylidenes, 4,5-dihydroimidazol-2-ylidenes and triazol-2-ylidenes are documented. Computational studies are used to explore the link between the shielding of the selenium centre and the electronic properties of the NHCs. Results show that δSe is correlated to the energy gap between a filled lone pair orbital on Se and the empty π* orbital corresponding to the Se–NHC bond. Bond energy decomposition analysis indicated no correlation between the orbital σ-contribution to bonding and the chemical shielding, while a good correlation was found between the π-contribution to bonding and the chemical shielding, confirming that this technique is indeed able to quantify the ability of NHCs to accept π-electron density. Calculations conducted on phosphinidene adducts yielded similar results. With the link between δSe and δP and π-back bonding ability clearly established, these compounds represent useful ways in which to fully understand and quantify this aspect of the electronic properties of NHCs.

 Please wait while we load your content...
Please wait while we load your content...