Assembly line termination in cylindrocyclophane biosynthesis: discovery of an editing type II thioesterase domain in a type I polyketide synthase†
Abstract
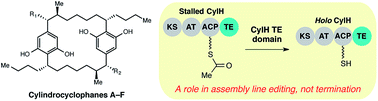
The termination step is an important source of structural diversity in polyketide biosynthesis. Most type I polyketide synthase (PKS) assembly lines are terminated by a thioesterase (TE) domain located at the C-terminus of the final module, while other PKS assembly lines lack a terminal TE domain and are instead terminated by a separate enzyme in trans. In cylindrocyclophane biosynthesis, the type I modular PKS assembly line is terminated by a freestanding type III PKS (CylI). Unexpectedly, the final module of the type I PKS (CylH) also possesses a C-terminal TE domain. Unlike typical type I PKSs, the CylH TE domain does not influence assembly line termination by CylI in vitro. Instead, this domain phylogenetically resembles a type II TE and possesses activity consistent with an editing function. This finding may shed light on the evolution of unusual PKS termination logic. In addition, the presence of related type II TE domains in many cryptic type I PKS and nonribosomal peptide synthetase (NRPS) assembly lines has implications for pathway annotation, product prediction, and engineering.
- This article is part of the themed collection: Editors’ Choice Collection

 Please wait while we load your content...
Please wait while we load your content...