Cobalt co-catalysis for cross-electrophile coupling: diarylmethanes from benzyl mesylates and aryl halides†
Abstract
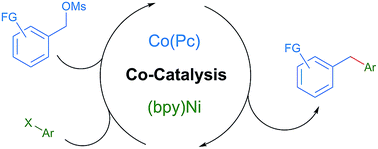
The nickel-catalyzed cross-coupling of aryl halides with alkyl radicals derived from alkyl halides has recently been extended to couplings with carbon radicals generated by a co-catalyst. In this study, a new co-catalyst, cobalt phthalocyanine (Co(Pc)), is introduced and demonstrated to be effective for coupling substrates not prone to homolysis. This is because Co(Pc) reacts with electrophiles by an SN2 mechanism instead of by the electron-transfer or halogen abstraction mechanisms previously explored. Studies demonstrating the orthogonal reactivity of (bpy)Ni and Co(Pc), applying this selectivity to the coupling of benzyl mesylates with aryl halides, and the adaptation of these conditions to the less reactive benzyl phosphate ester and an enantioconvergent reaction are presented.

 Please wait while we load your content...
Please wait while we load your content...