C–H arylation of triphenylene, naphthalene and related arenes using Pd/C†
Abstract
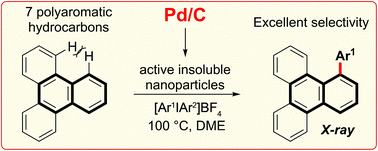
A highly selective arylation of a number of polyaromatic hydrocarbons (PAHs) with aryliodonium salts and Pd/C as the only reagent is reported. The first C–H functionalization of triphenylene is explored, and proceeds at the most sterically hindered position. This non-chelate assisted C–H functionalization extends the reactivity profile of Pd/C and provides controlled access to π-extended PAHs, an important aspect of work towards the preparation of nanographenes. Mechanistic studies suggest in situ formation of catalytically active insoluble nanoparticles, and that the reaction likely proceeds via a Pd(0)/Pd(II) type reaction manifold.

 Please wait while we load your content...
Please wait while we load your content...